Results
At baseline, 382 subjects (57.6%) were taking DMARDs and/or glucocorticoids:
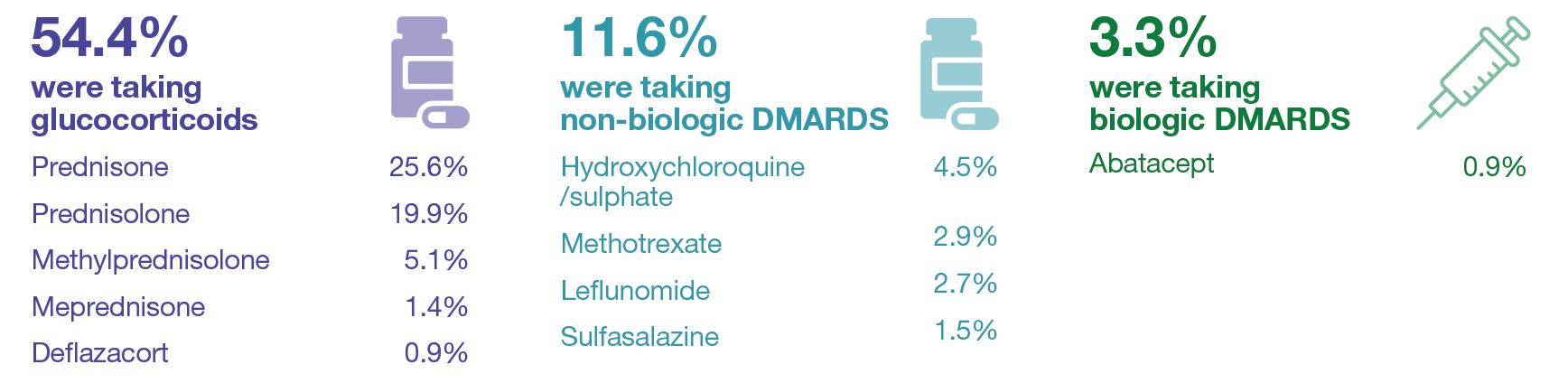
Therapies taken by ≥5 patients (0.8%) are shown.
Baseline characteristics of subgroups by use of DMARDs and/or glucocorticoids
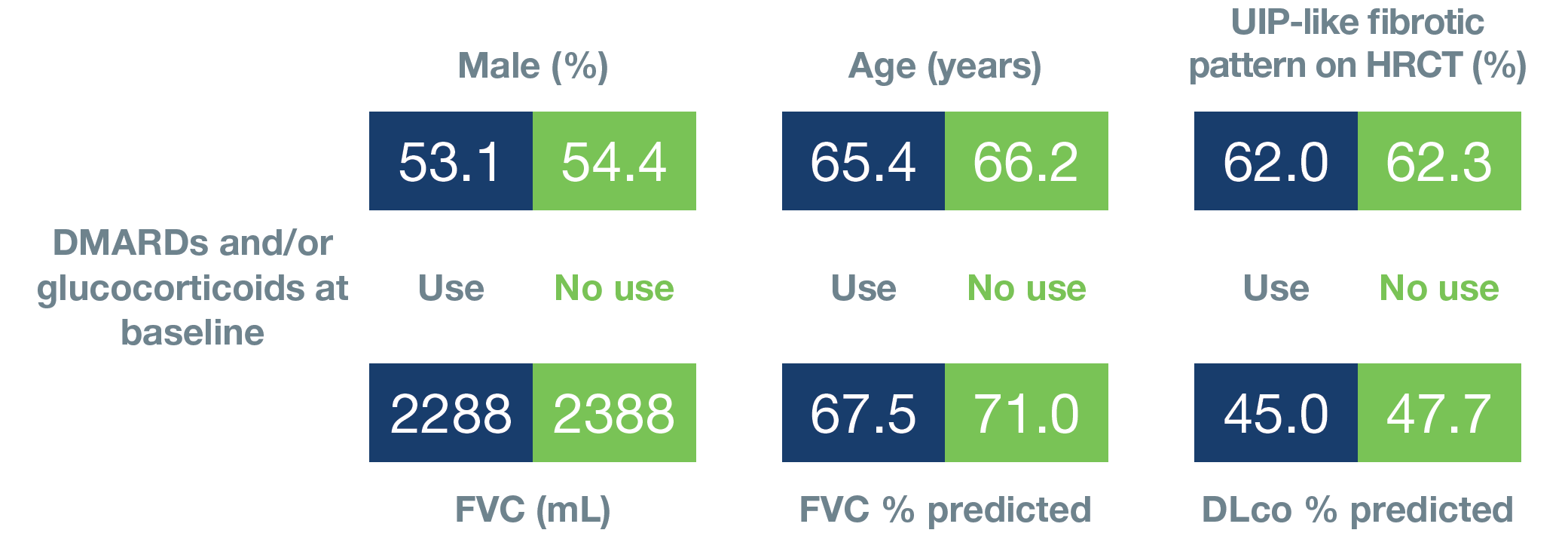
Mean or % of subjects
ILD diagnoses in subgroups by use of DMARDs and/or glucocorticoids at baseline
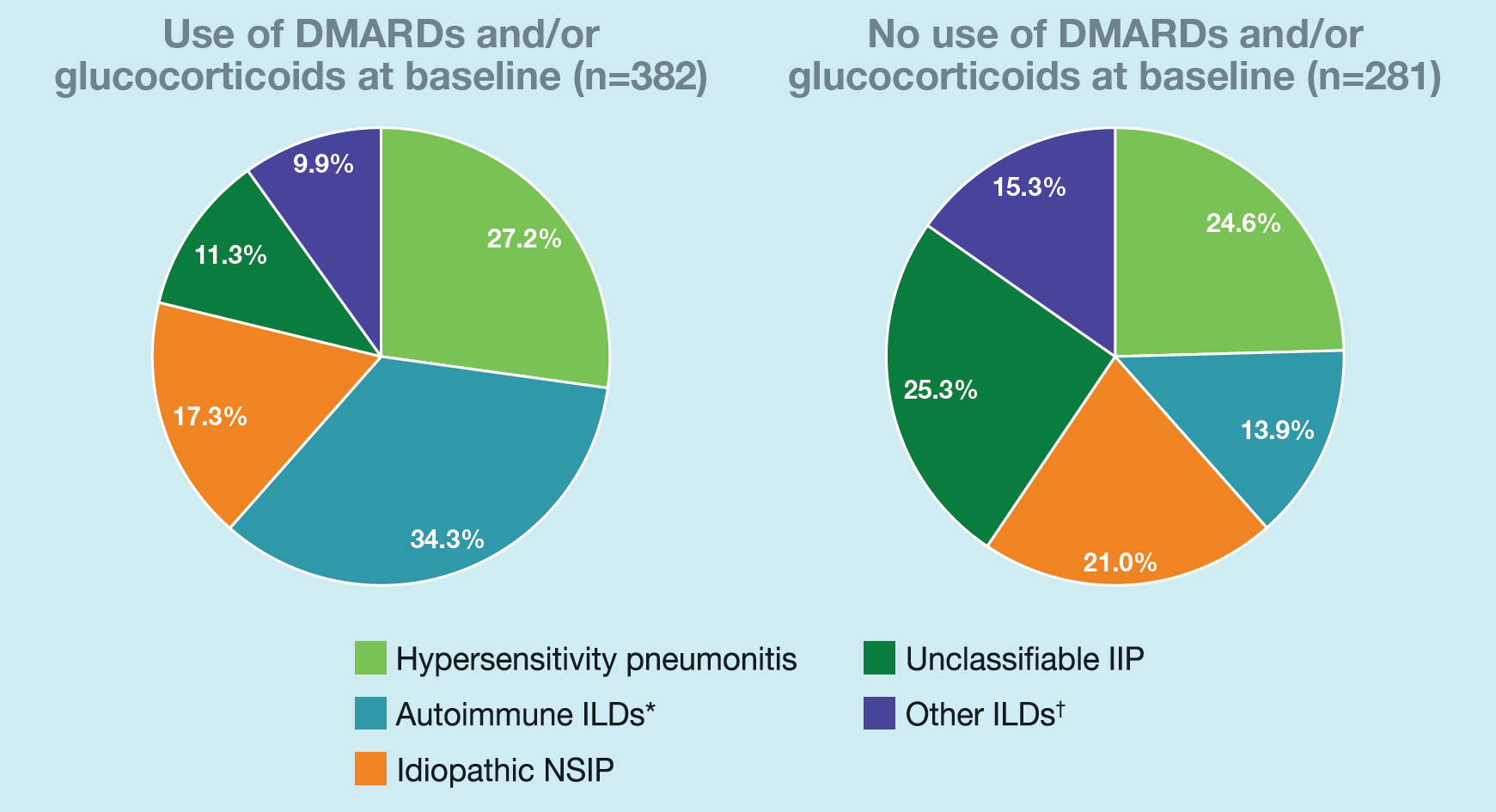
*Included rheumatoid arthritis-associated ILD, systemic sclerosis-associated ILD, mixed connective tissue disease-associated ILD, plus subjects with an autoimmune disease noted in the “Other fibrosing ILDs” category of the case report form. †Included sarcoidosis, exposure-related ILDs and selected terms in the “Other fibrosing ILDs” category of the case report form. NSIP, non-specific interstitial pneumonia.
Rate of decline in FVC (mL/year) over 52 weeks
- In the placebo group, the rate of FVC decline was numerically greater in subjects who used DMARDs and/or glucocorticoids at baseline (Figure 1).
- Nintedanib reduced the rate of FVC decline versus placebo both in subjects who did and did not use DMARDs and/or glucocorticoids at baseline. The interaction p-value did not indicate a differential treatment effect of nintedanib between these subgroups (Figure 1).
- Similar results were observed in subgroups by use of DMARDs (only) and glucocorticoids (only) (Figures 2 and 3).
Figure 1. Rate of decline in FVC (mL/year) over 52 weeks in subgroups by use of DMARDs and/or glucocorticoids at baseline
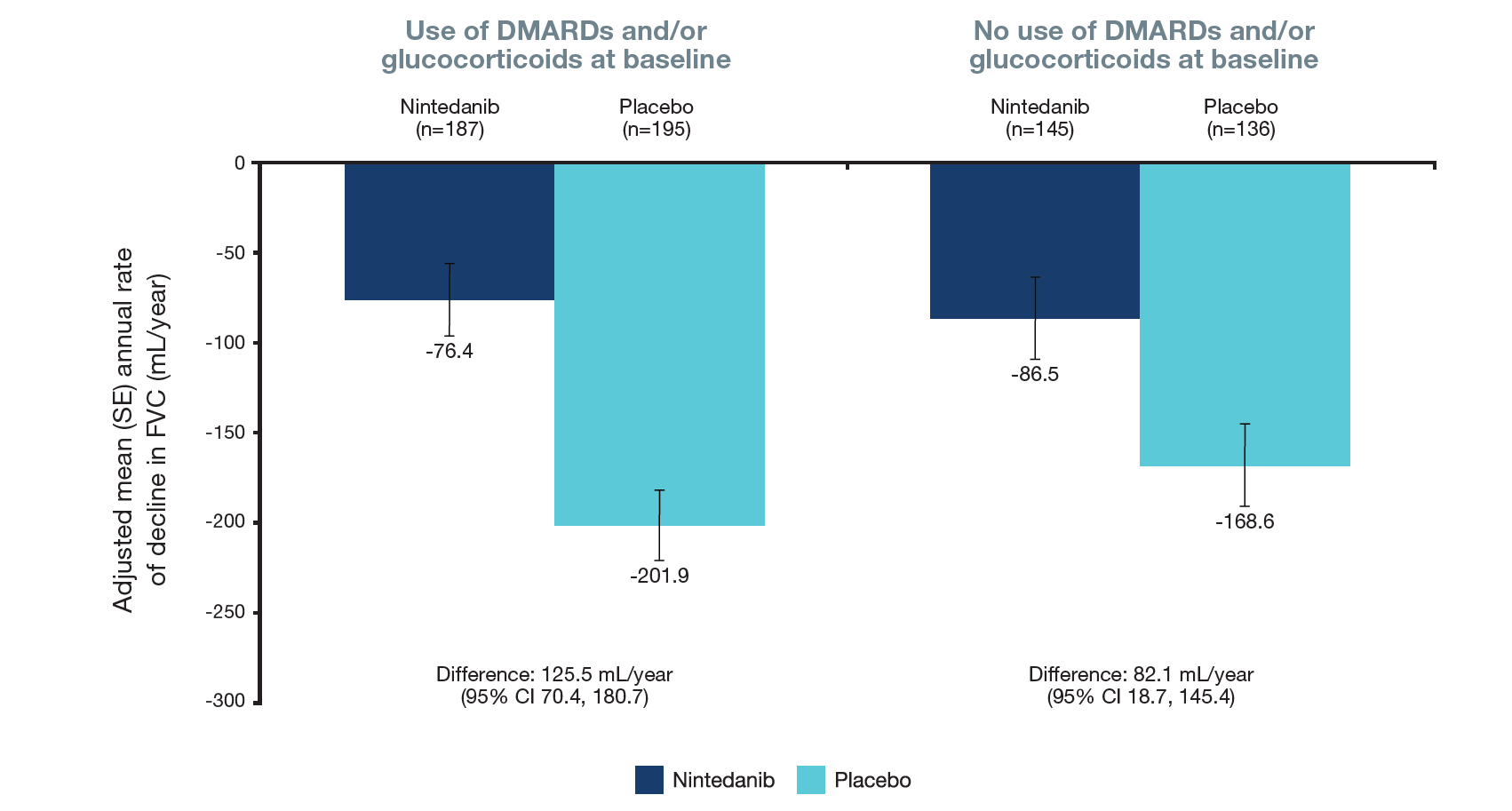
Treatment-by-subgroup-by time interaction p=0.31
Figure 2. Rate of decline in FVC (mL/year) over 52 weeks in subgroups by use of DMARDs at baseline
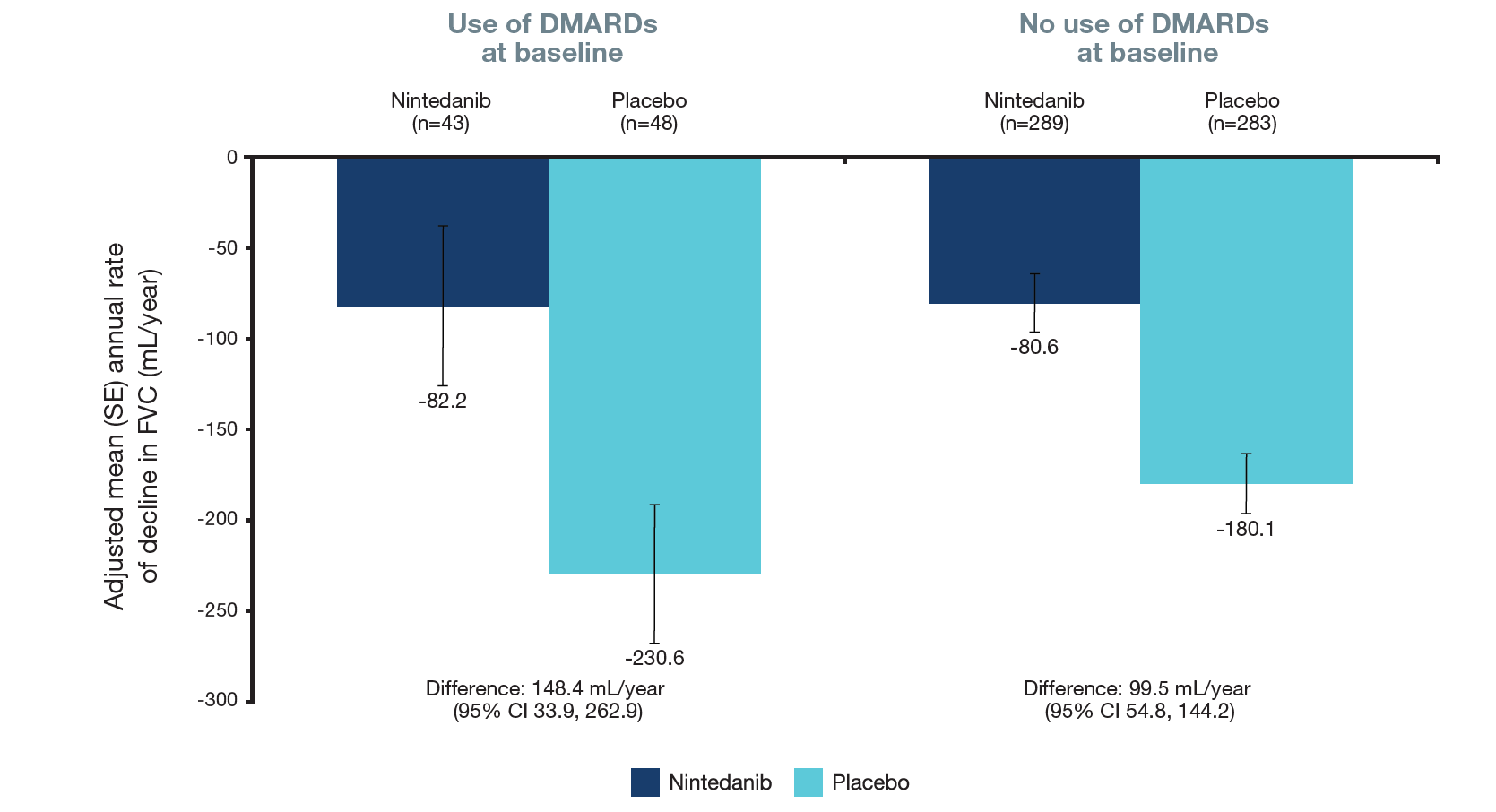
Treatment-by-subgroup-by time interaction p=0.44
Figure 3. Rate of decline in FVC (mL/year) in subgroups by use of glucocorticoids at baseline
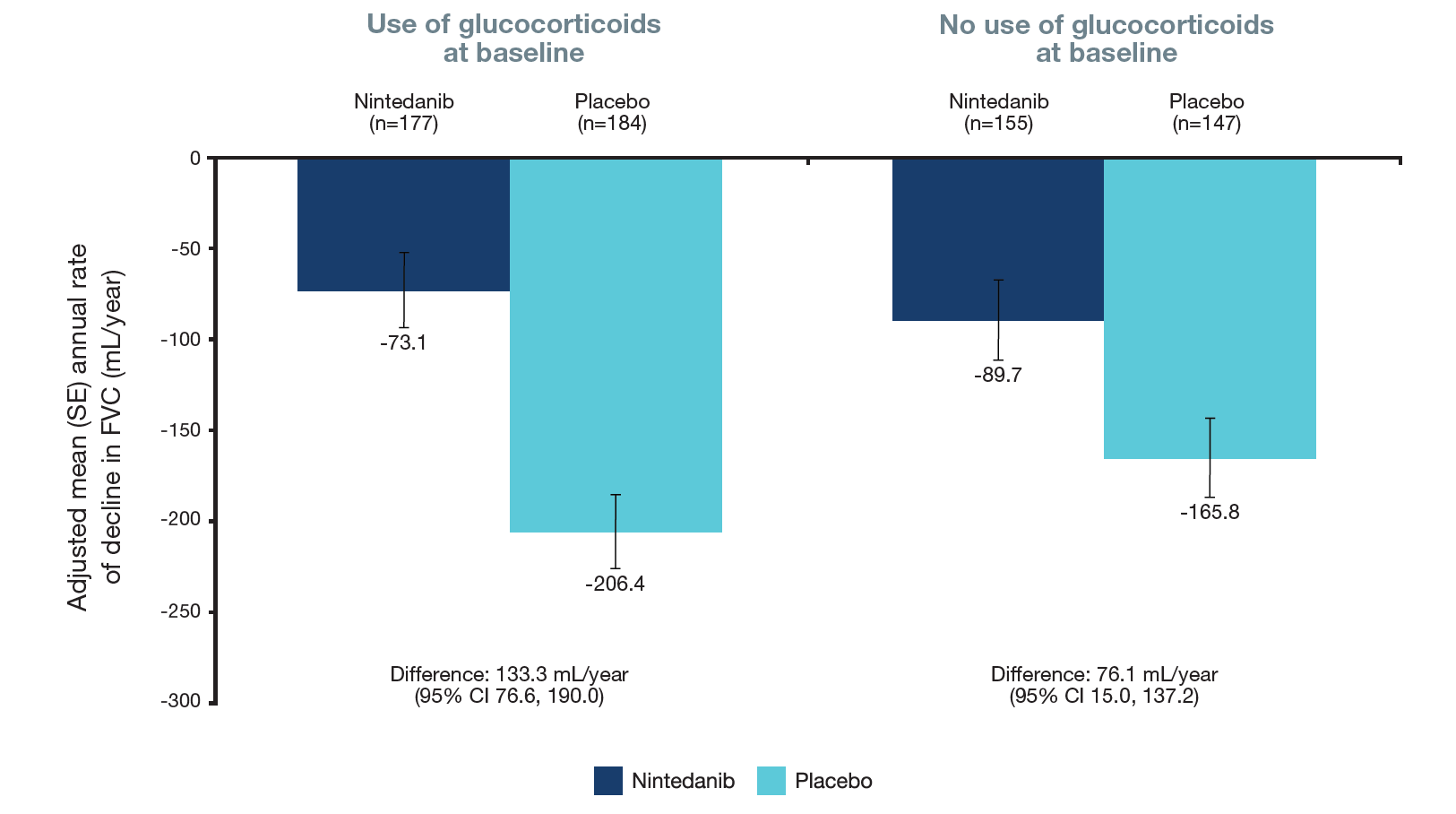
Treatment-by-subgroup-by time interaction p=0.18
Adverse events
- The adverse event profile of nintedanib was generally consistent between subgroups by use of DMARDs and/or glucocorticoids at baseline (Figures 4 and 5).
Figure 4. Most frequent adverse events (reported irrespective of causality) in subgroups by use of DMARDs and/or glucocorticoids at baseline
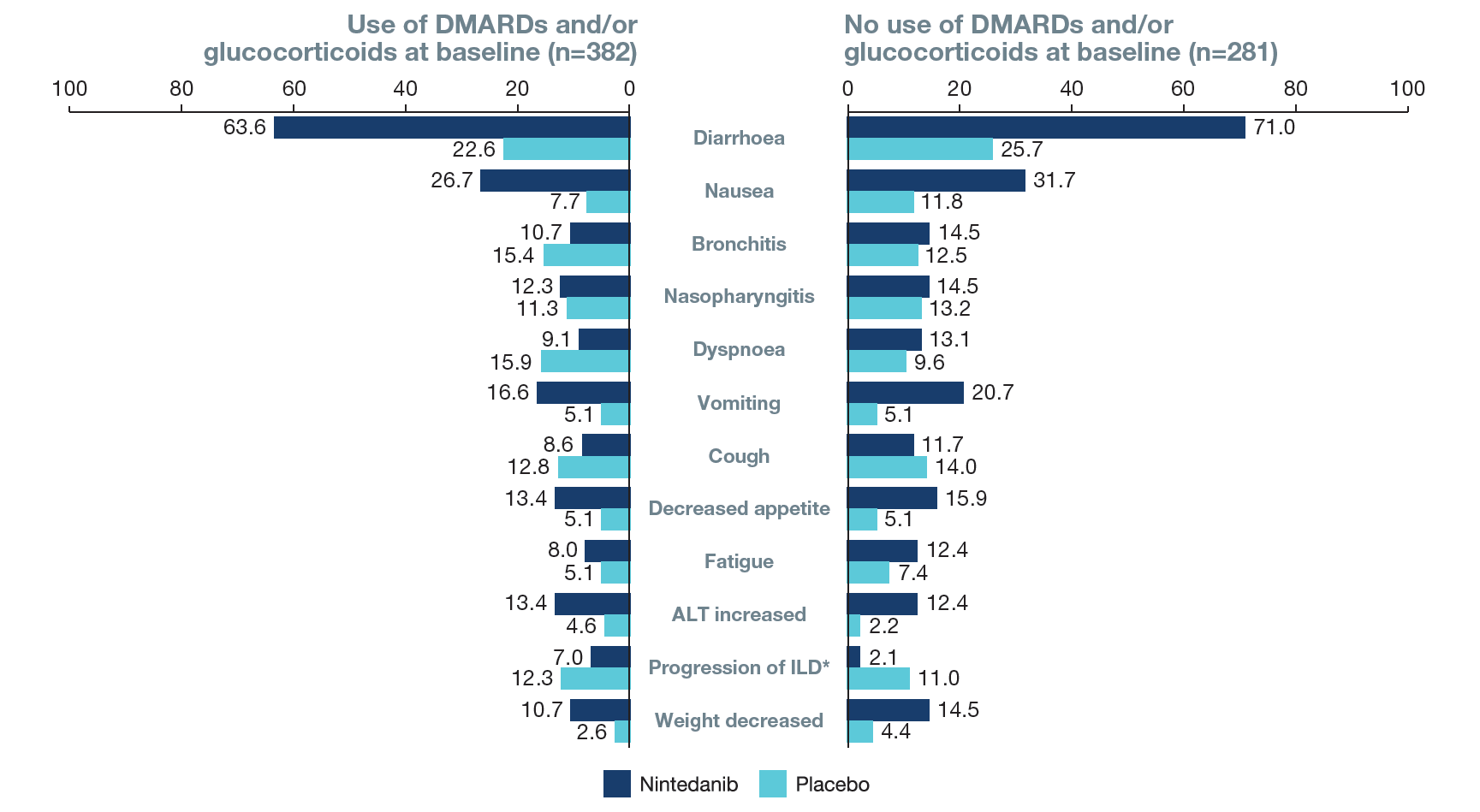
Adverse events (AEs) were coded using MedDRA preferred terms. Data are % of subjects with ≥1 such adverse event, reported over 52 weeks (or until 28 days after last trial drug intake in subjects who discontinued trial drug before week 52). AEs reported in >12% of subjects in either of the subgroups are shown.
*Based on MedDRA preferred term “interstitial lung disease”. ALT, alanine aminotransferase.
Figure 5. Proportions of subjects with adverse events leading to discontinuation of trial drug
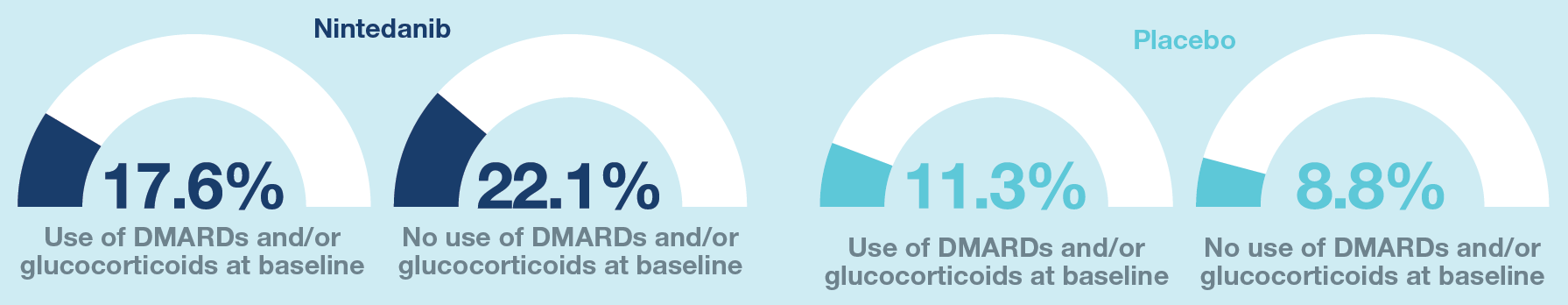
Data are % of subjects with ≥1 such adverse event reported over 52 weeks.