Contents
Methods
Trial design1
- Subjects in the INBUILD trial had an ILD other than IPF, diagnosed according to the investigator’s usual clinical practice; reticular abnormality with traction bronchiectasis (with or without honeycombing) of >10% extent on HRCT; FVC ≥45% predicted; diffusing capacity for carbon monoxide (DLco) ≥30%–<80% predicted.
- Subjects met ≥1 of the following criteria for ILD progression in the 24 months before screening, despite management deemed appropriate in clinical practice:
Image
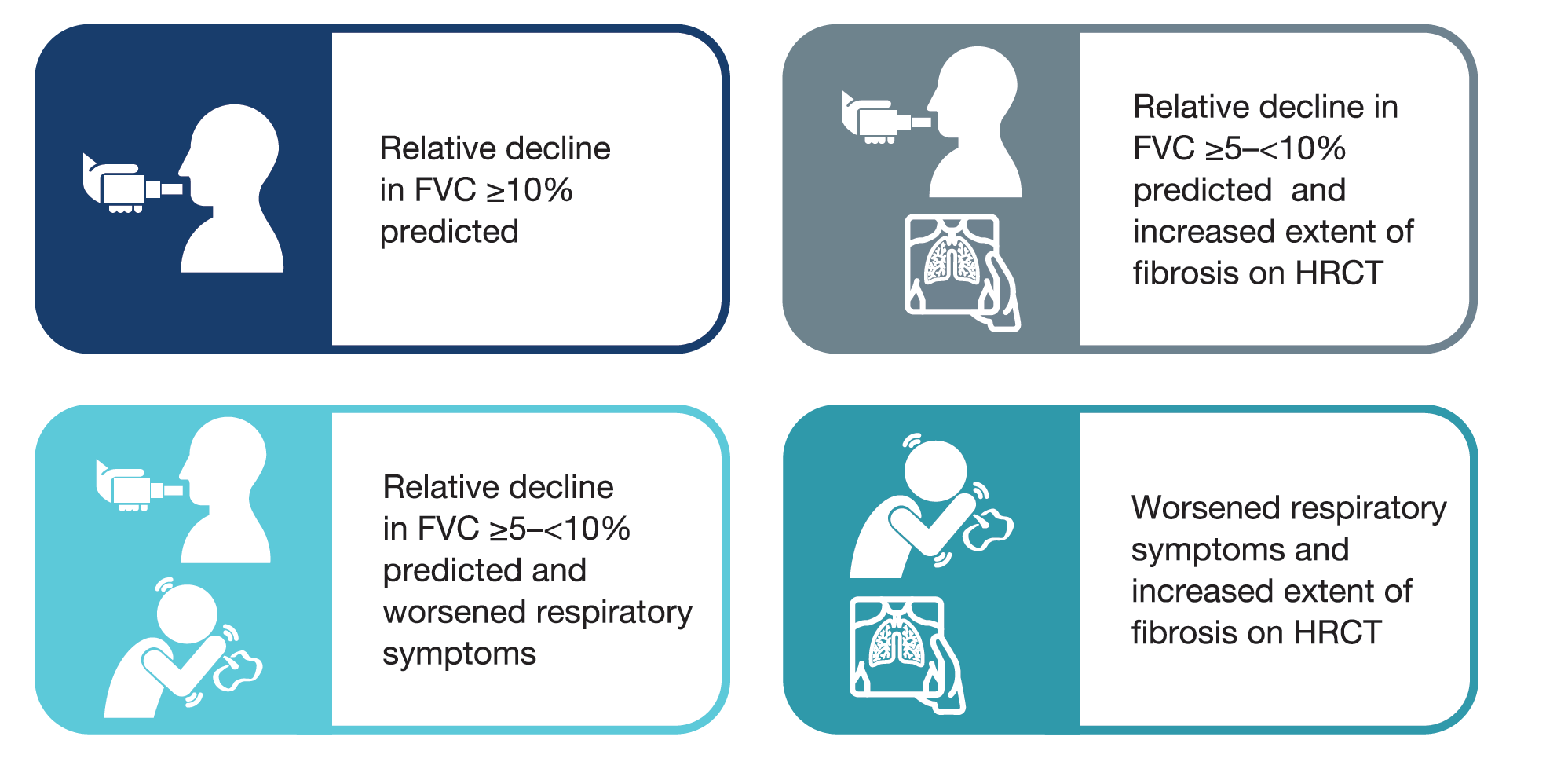
- Subjects were randomised to receive nintedanib or placebo, stratified by fibrotic pattern on HRCT (usual interstitial pneumonia [UIP]-like fibrotic pattern or other fibrotic patterns).
- Patients taking stable doses of medications to treat autoimmune rheumatic diseases were eligible to participate, but patients taking azathioprine; cyclosporine; mycophenolate mofetil, tacrolimus; oral glucocorticoids >20 mg/day; the combination of oral glucocorticoids, azathioprine and N-acetylcysteine; cyclophosphamide; or rituximab were not eligible.
- Investigators were asked not to consider patients with autoimmune disease that was managed using any of these therapies for participation in the trial. Patients who took these therapies to treat their ILD, and whose ILD was progressing, could participate in the trial if the restricted therapy was discontinued.
- Apart from the restricted therapies listed above, there was no limit on the use of stable doses of biologic or non-biologic DMARDs.
- Initiation of the above therapies was allowed after 6 months of trial treatment in subjects with deterioration of ILD or autoimmune disease.
Analyses
- We assessed the rate of decline in FVC (mL/year) over 52 weeks in subgroups by use of DMARDs and/or glucocorticoids at baseline.
- DMARDs were identified based on the WHO standardised drug grouping (SDG) plus baricitinib and excluding denosumab. Glucocorticoids (with oral, intravenous [IV], IV bolus, IV drip, or intramuscular administration) were identified based on the WHO SDG.
- Interaction p-values were calculated to assess potential heterogeneity in the treatment effect of nintedanib versus placebo across the subgroups. No adjustment for multiplicity was made.
- Adverse events are presented descriptively.