Contents
Methods
Trial design5
- Subjects in the INBUILD trial had an ILD other than IPF, diagnosed according to the investigator’s usual clinical practice; reticular abnormality with traction bronchiectasis (with or without honeycombing) of >10% extent on HRCT based on central review; FVC ≥45% predicted; diffusion capacity of the lung for carbon monoxide (DLco) ≥30%–<80% predicted.
- Subjects met ≥1 of the following criteria for ILD progression in the 24 months before screening, despite management deemed appropriate in clinical practice:
Image
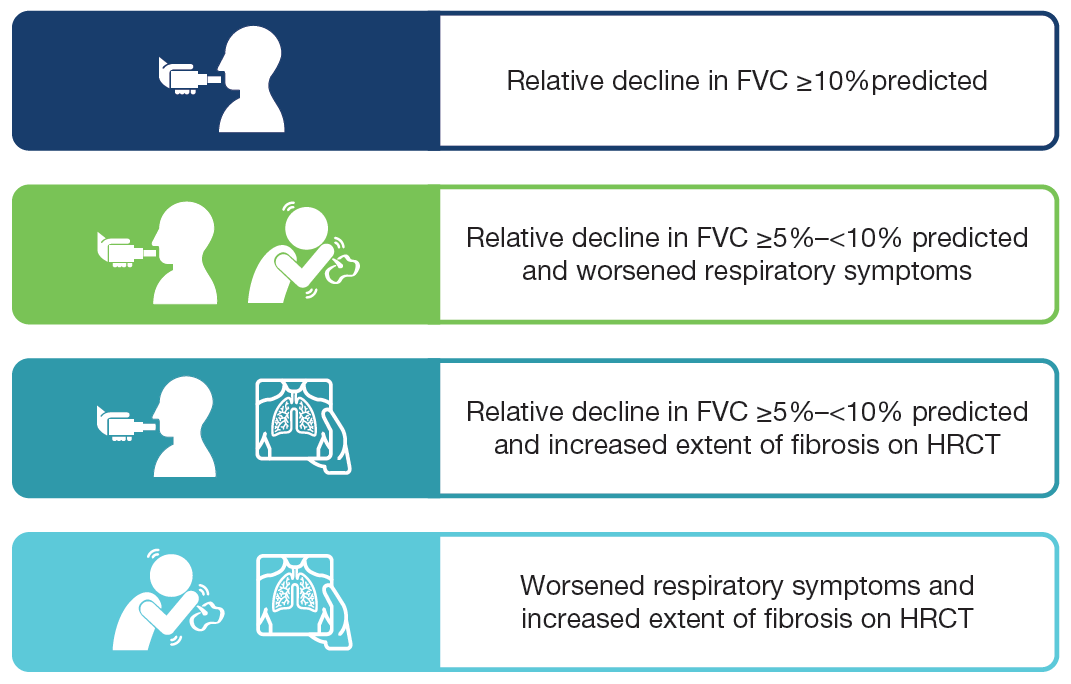
- Subjects were randomised to receive nintedanib or placebo, stratified by fibrotic pattern on HRCT (usual interstitial pneumonia [UIP]-like fibrotic pattern or other fibrotic patterns).
Analyses
- We assessed the rate of decline in FVC (mL/year) over 52 weeks in subgroups by FVC % predicted at baseline (≤50%, >50%–≤70%, >70% predicted).
- Interaction p-values were calculated to assess potential heterogeneity in the treatment effect of nintedanib versus placebo across the subgroups. No adjustment for multiplicity was made.
- Adverse events are presented descriptively.